The clinical course of schizophrenia is often marked by recurrent relapse, which can have significant consequences for patients, including the development of treatment resistance—which can emerge as early as the first relapse—as well as social challenges such as arrests and homelessness.1-5 Long-acting injectable (LAI) therapies play a role in schizophrenia treatment by delivering sustained therapeutic doses and creating fewer opportunities to miss a dose, thereby reducing the risk of relapse.6,7
UZEDY, an LAI formulation of the antipsychotic risperidone, has been clinically proven to reduce the risk of relapse in patients with schizophrenia.8 It utilizes the innovative drug delivery technology, SteadyTeq™, to subcutaneously deliver risperidone steadily over the course of the treatment interval.9,10
The safety and efficacy of UZEDY in patients with schizophrenia was evaluated in the RISE trial, a randomized, double-blind, placebo-controlled, relapse-prevention study. RISE was designed to compare UZEDY Q1M (once monthly) or UZEDY Q2M (once every 2 months) vs placebo, but not vs each other.
With that in mind, patients treated with UZEDY Q1M experienced an 80% reduction in risk of relapse vs patients treated with placebo (Figure 1).8 UZEDY Q2M reduced the risk of relapse vs placebo by 62.5%.8 The RISE trial was not designed to compare patients treated with UZEDY Q1M vs UZEDY Q2M.
Figure 1. Reduction in Risk of Relapse in RISE
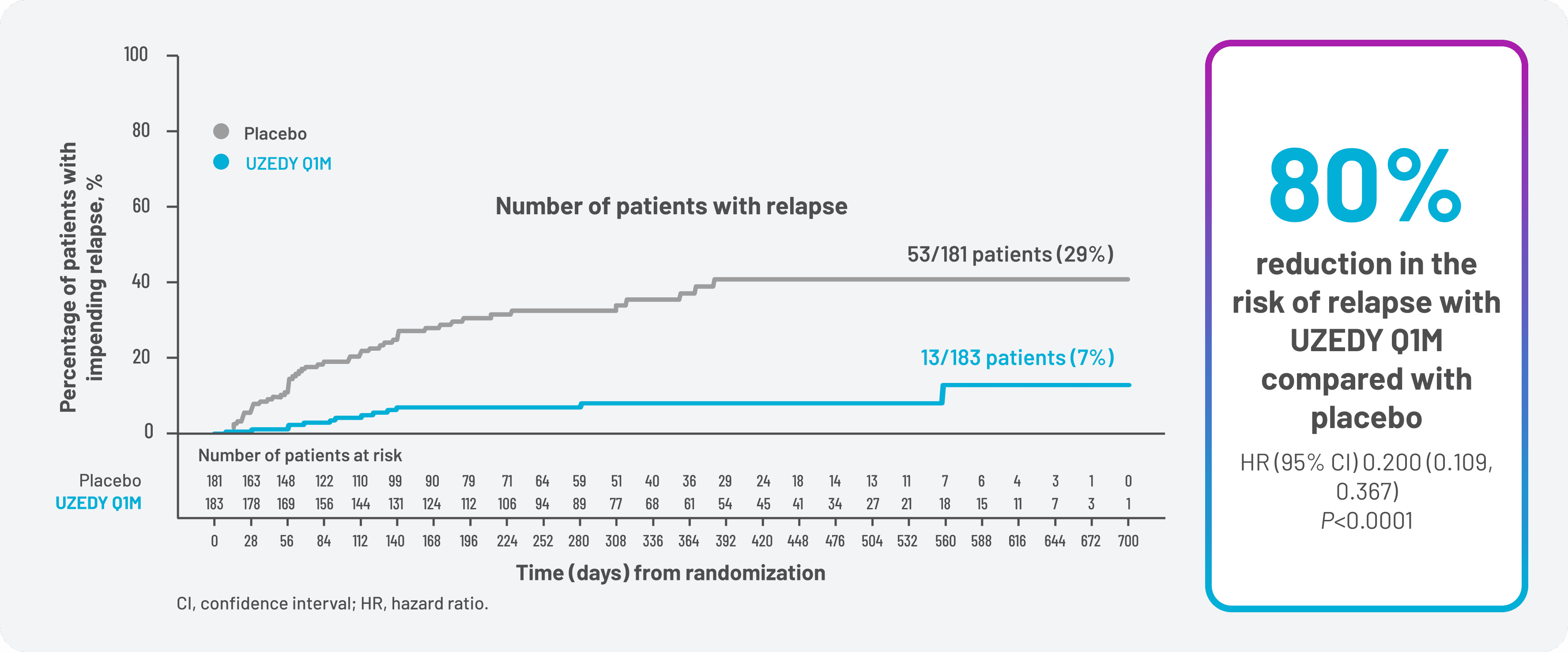
For patients treated with UZEDY Q1M, relapse prevention was sustained at 6 months with a relapse-free rate of 93% vs 72% for patients treated with placebo. At 1 year, the relapse-free rate for patients treated with UZEDY Q1M was 92% vs 63% for patients treated with placebo.10 Sustained relapse prevention was also demonstrated in patients treated with UZEDY Q2M, with relapse-free rates at 6 months of 89% vs 72% for patients treated with placebo, and 87% vs 63% for patients treated with placebo at 1 year.10 Relapse-free rate at 6 months was a prespecified exploratory endpoint, and relapse-free rate at 1 year was a post hoc analysis. No determination of statistical significance can be made from exploratory or post hoc analyses, and no conclusions about efficacy should be drawn.
The safety profile of oral risperidone in adults with schizophrenia has been well characterized. The safety profile of UZEDY for patients with schizophrenia is based on adequate and well-controlled studies of oral risperidone, and the safety profile of UZEDY is expected to be similar to that of corresponding oral risperidone doses of 2 to 5 mg daily.11
The most common adverse reactions with oral risperidone (>5% and twice placebo) were parkinsonism, akathisia, dystonia, tremor, sedation, dizziness, anxiety, blurred vision, nausea, vomiting, upper abdominal pain, stomach discomfort, dyspepsia, diarrhea, salivary hypersecretion, constipation, dry mouth, increased appetite, increased weight, fatigue, rash, nasal congestion, upper respiratory tract infection, nasopharyngitis, and pharyngolaryngeal pain.11
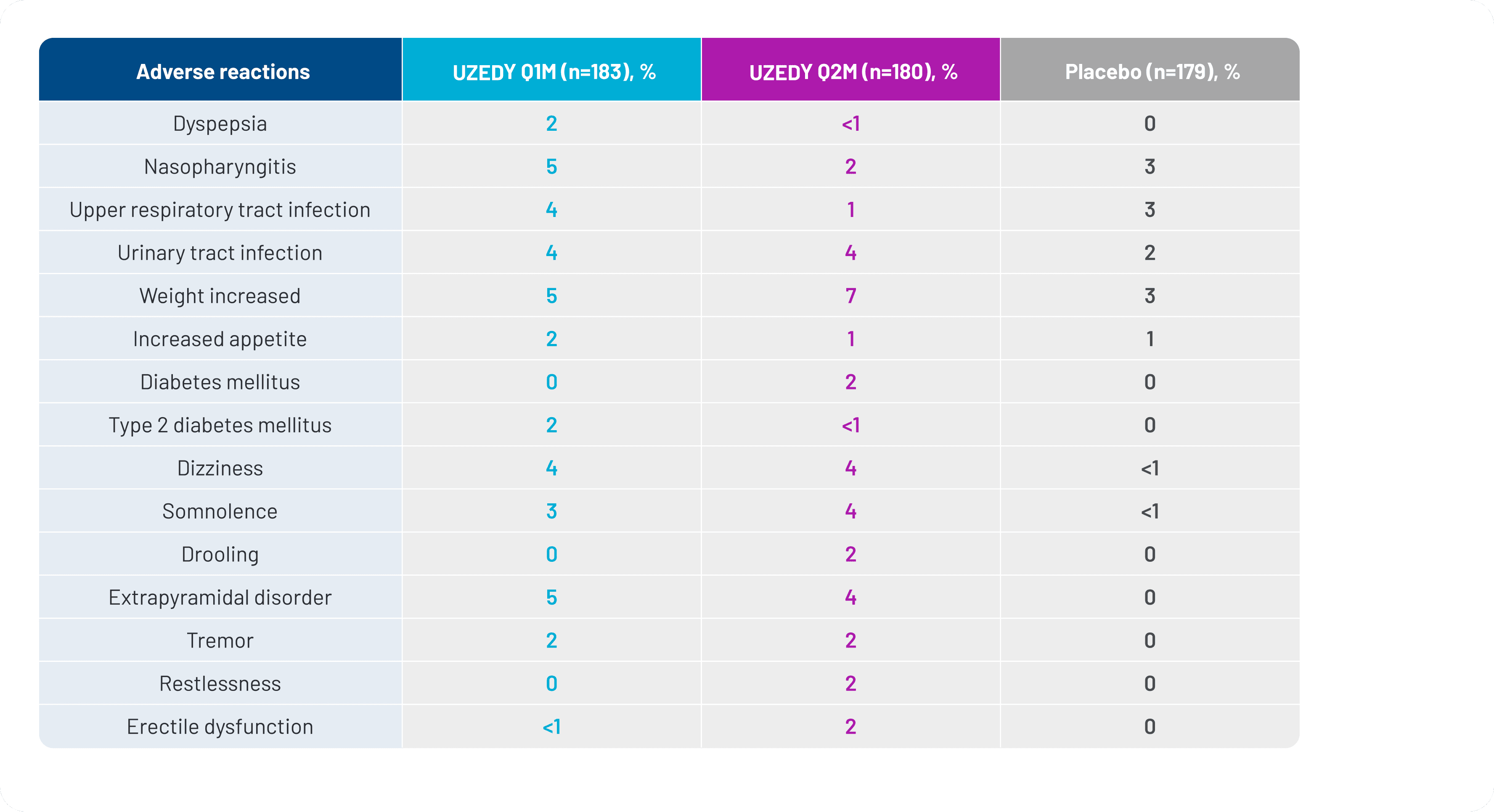
The systemic safety profile for UZEDY is consistent with the known safety profile of risperidone. The adverse events occurring in 5% or more of patients taking UZEDY and more frequently than with placebo were nasopharyngitis, weight increase, and extrapyramidal disorder.8,10 Adverse reactions occurring in ≥2% of patients in the RISE clinical trial are shown in Figure 2.
Figure 2. Adverse Reactions Occurring in ≥2% of Patients in the RISE Clinical Trial
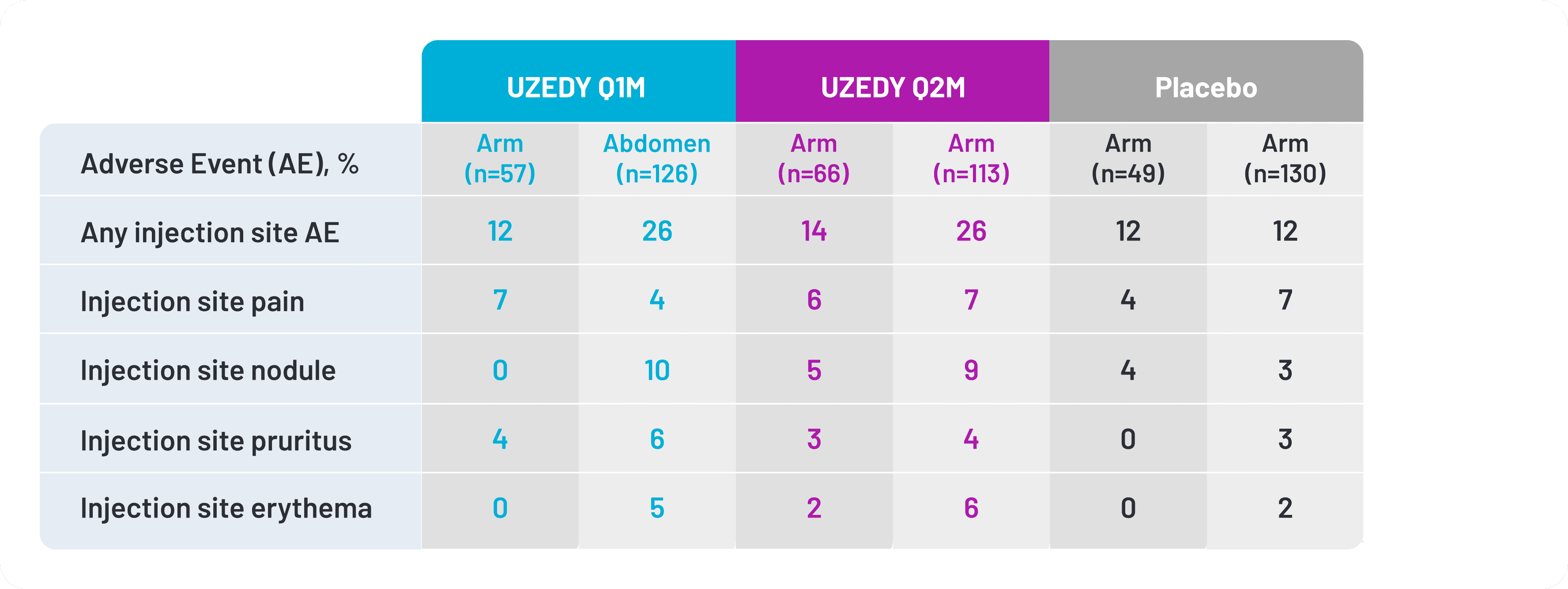
Most injection site reactions were mild or moderate.12 The most common injection site reactions with UZEDY (≥5% and greater than placebo) were pruritus and nodule.11 Injection site reactions were more frequent in the abdomen than in the arm.10 In the RISE clinical trial, 32% of patients received UZEDY in the upper arm and 68% received UZEDY in the abdomen.10 Injection site reactions reported in the RISE clinical trial are shown in Figure 3.12
Figure 3. Injection Site Reactions Occurring in the RISE Clinical Trial
UZEDY has a number of pharmacokinetic (PK), administrative, and usage features that clinicians and patients may consider when choosing an LAI.
PK studies showed that a single dose of UZEDY reached therapeutic plasma concentrations within 6 to 24 hours, allowing treatment initiation or reinitiation with a single injection; no oral supplementation or loading dose is required.8,11 Therapeutic plasma concentrations are sustained for the duration of the dosing interval, and for all doses, steady‑state levels of risperidone and 9-hydroxyrisperidone were approached within 2 months of UZEDY initiation, which is after 2 injections for UZEDY Q1M and 1 injection for UZEDY Q2M.11
The dosing interval and dosage strength of UZEDY can also be tailored to the individual needs of the patient. UZEDY can be dosed at 1- or 2-month intervals from initiation and is available in 4 strengths at each dosing interval, corresponding to 2 mg to 5 mg oral risperidone.11
Figure 4. Needle Sizes of Available LAIs
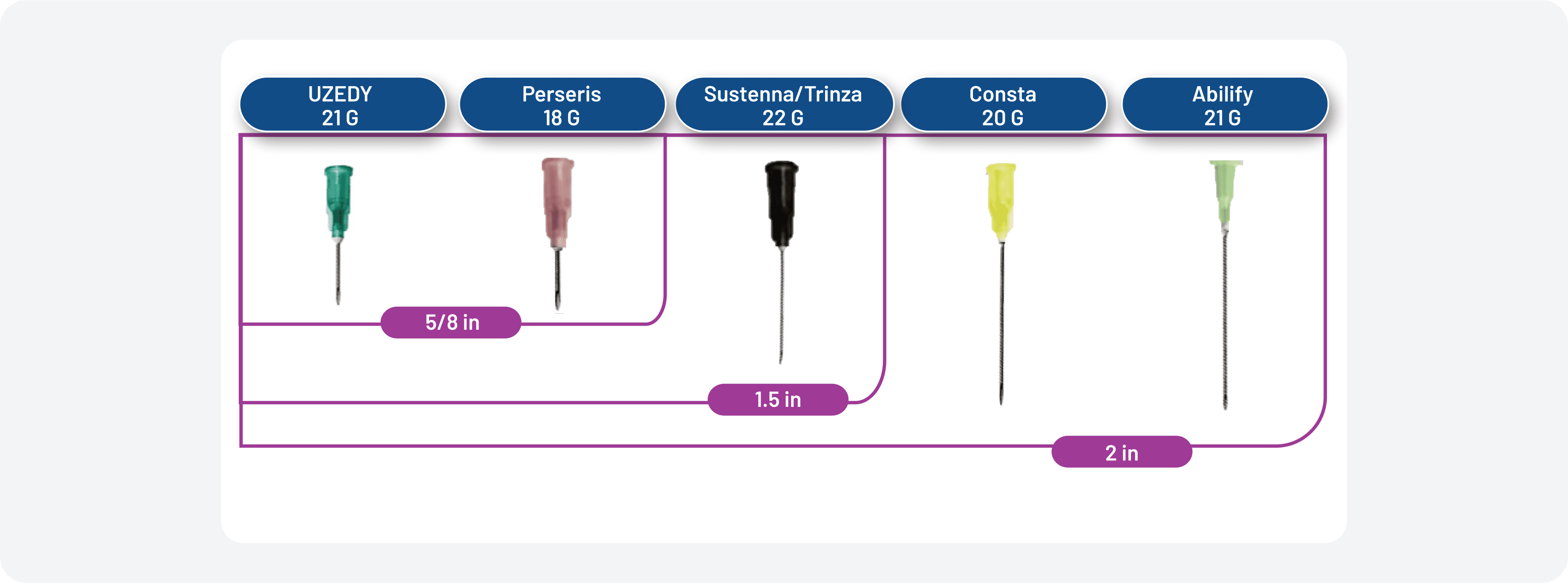
UZEDY comes in a prefilled syringe with a 5/8-inch, 21-gauge needle. The needle sizes of other available LAIs can be seen in Figure 4.11,13-17 UZEDY should be administered subcutaneously in the abdomen or upper arm by a healthcare professional.11
Data were collected from 63 patients, 24 physicians, and 25 nurses in a prospective, cross-sectional companion survey assessing the perceptions regarding ease of use and satisfaction with UZEDY. The survey was administered after a minimum of 2 experiences prescribing, administering, or receiving UZEDY.18 89% of patients found UZEDY easy to receive, and 90% reported that they would choose to remain on UZEDY over their previous schizophrenia medication.18
Determining whether to switch a patient from another LAI to UZEDY relies on careful consideration of multiple factors, including patient preference, scheduling convenience, and concerns about tolerability or symptom breakthrough. Because switching between antipsychotics is a potentially destabilizing event that may lead to the development of psychotic symptoms or other clinically relevant adverse events, it is up to clinician discretion to determine if and when switching is clinically appropriate for an individual patient.19
Clinical trials studied switching from oral risperidone to UZEDY, but switching to UZEDY from other LAIs or molecules was not directly studied, and no clear guidelines exist regarding switching from one LAI to another.11,19
With that in mind, Teva Pharmaceuticals, Inc. conducted population pharmacokinetic (popPK) modeling to simulate dosing conversions and strategies for switching adult patients from intramuscular paliperidone palmitate once monthly (PP1M) to UZEDY.20 Pharmacokinetically comparable doses of oral risperidone, PP1M, and UZEDY based on popPK modeling are shown in Figure 5.
Figure 5. Pharmacokinetically Comparable Doses of Oral Risperidone, PP1M, and UZEDY
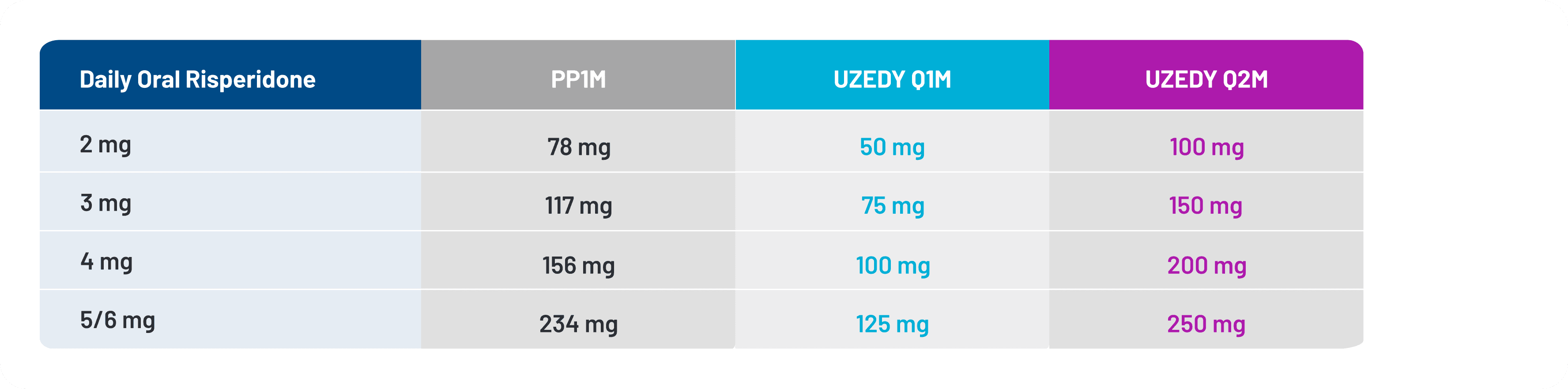
The highest dose strength available for UZEDY is comparable to 5 mg of oral risperidone.11 Paliperidone is the active metabolite of risperidone; however, for patients who have never taken risperidone, it is important to establish its tolerability before initiating UZEDY.11
This simulation study utilized published popPK models for PP1M and UZEDY. Using virtual populations of 5000 patients, total active moiety, or TAM, concentration-time profiles were simulated to predict PK exposures for TAM when switching to either UZEDY Q1M or UZEDY Q2M 4 weeks after the last injection of PP1M, which was assumed to be at steady state.20
One switching strategy that was simulated using this method was for a 1:1 steady‑state, comparable dose switch from PP1M to either UZEDY Q1M or Q2M for a hypothetical patient currently taking a 234 mg dose of PP1M. The comparable doses for UZEDY Q1M and UZEDY Q2M were 125 mg and 250 mg, respectively, to be administered 4 weeks after the last dose of PP1M.20
Figure 6. TAM Concentration Simulation
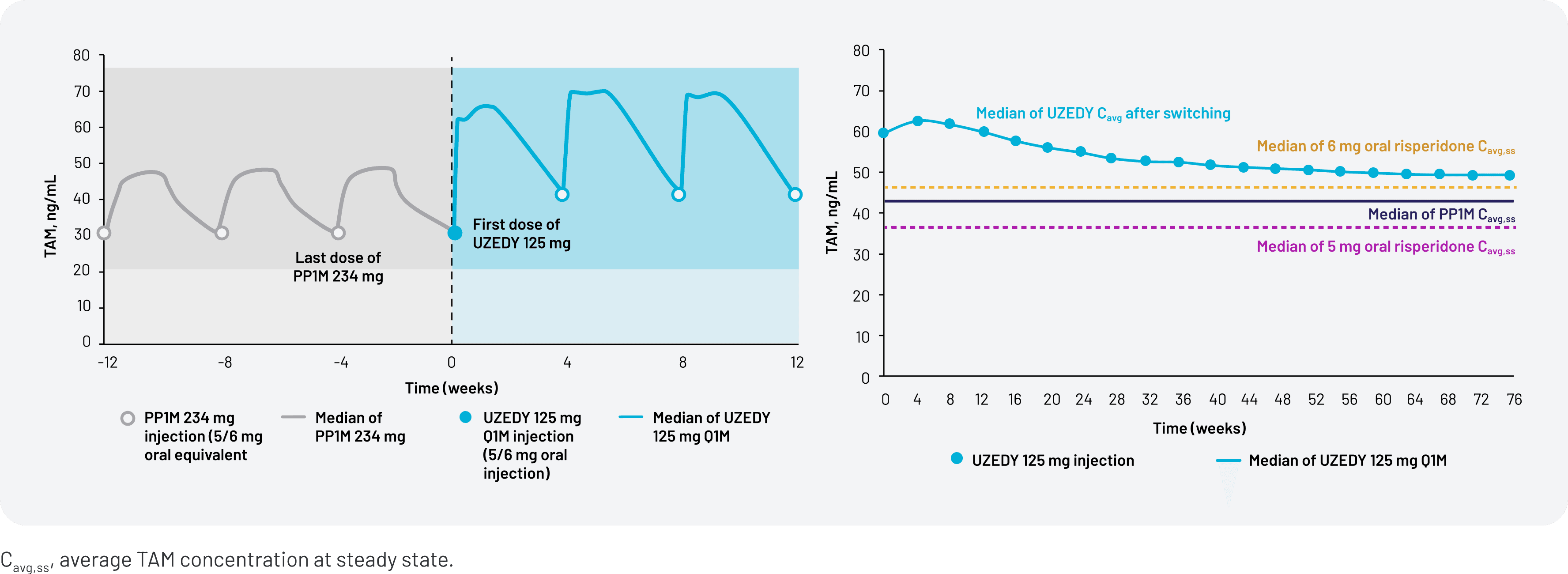
Figure 6 shows the simulated TAM concentration when switching from PP1M 234 mg to UZEDY Q1M 125 mg (left) and the average TAM concentrations of UZEDY vs PP1M over time (right).20 While these data represent a potential strategy when switching to UZEDY from PP1M, the relationship between pharmacokinetics and efficacy has not been established. Because there have been no head-to-head studies conducted comparing UZEDY with other LAI antipsychotics, no comparisons regarding efficacy, safety, or other clinical outcomes can be made. These popPK simulation data are intended to provide guidance when switching patients to UZEDY, but clinical outcomes should be monitored and the dose adjusted as appropriate based on the provider’s judgment.
In addition to the popPK simulation data for switching from PP1M to UZEDY, popPK studies to evaluate switch strategies from other LAIs to UZEDY have also been conducted. Any questions regarding these data can be directed to Teva Medical Affairs.
- Relapse reduction has been demonstrated in patients with schizophrenia who were treated with UZEDY Q1M and Q2M vs placebo8
- The safety profile of UZEDY was consistent with that of oral risperidone8,11
- When deemed appropriate by a clinician, patients taking PP1M may be switched to UZEDY by initiating a single, pharmacokinetically comparable dose of UZEDY 4 weeks after their last PP1M injection19,20
- For patients with schizophrenia, there are many factors to consider when selecting an LAI, and UZEDY has many features related to efficacy, safety, PK, and usage that clinicians and patients may consider when choosing a treatment or switching from another LAI to UZEDY8,19
- Kane JM, Correll CU. Optimizing treatment choices to improve adherence and outcomes in schizophrenia. J Clin Psychiatry. 2019;80(5):IN18031AH1C.
- Alvarez-Jiminez M, Priede A, Hetrick SE, et al. Risk factors for relapse following treatment for first episode psychosis: a systematic review and meta-analysis of longitudinal studies. Schizophr Res. 2012;139(1-3):116-128.
- Nasrallah HA. 10 devastating consequences of psychotic relapses. Curr Psychiatry. 2021;20(5):9-12.
- Emsley R, Oosthuizen P, Koen L, et al. Comparison of treatment response in second-episode versus first-episode schizophrenia. J Clin Psychopharmacol. 2013;33(1):80-83.
- Takeuchi H, Siu C, Remington G, et al. Does relapse contribute to treatment resistance? Antipsychotic response in first- vs. second-episode schizophrenia. Neuropsychopharmacology. 2019;44(6):1036-1042.
- Blackwood C, Sanga P, Nuamah I, et al. Patients’ preference for long-acting injectable versus oral antipsychotics in schizophrenia: results from the patient-reported Medication Preference Questionnaire. Patient Prefer Adherence. 2020;14:1093-1102.
- American Psychiatric Association. The American Psychiatric Association Practice Guideline for the Treatment of Patients With Schizophrenia. 3rd ed. Washington, DC: American Psychiatric Association; 2021.
- Kane JM, Harary E, Eshet R, et al. Efficacy and safety of TV-46000, a long-acting, subcutaneous, injectable formulation of risperidone, for schizophrenia: a randomised clinical trial in the USA and Bulgaria. Lancet Psychiatry. 2023;10(12):934-943.
- Perlstein I, Merenlender Wagner A, Gomeni R, et al. Population pharmacokinetic modeling and simulation of TV-46000: a long-acting injectable formulation of risperidone. Clin Pharmacol Drug Dev. 2022;11(7):865-877.
- Data on file. Parsippany, NJ: Teva Neuroscience, Inc.
- UZEDY® (risperidone) extended-release injectable suspension Current Prescribing Information. Parsippany, NJ: Teva Neuroscience, Inc.
- Correll CU, Kane JM, Suett M, et al. Efficacy and safety of TV-46000, subcutaneous long-acting risperidone, by injection site (upper arm/abdomen): post hoc analysis of the RISE study. Presented at: Psych Congress; October 29-November 1, 2021; San Antonio, TX.
- Risperdal Consta® (risperidone) long-acting injection. Prescribing Information. Titusville, NJ: Janssen Pharmaceuticals, Inc.
- PERSERIS® (risperidone) extended-release injectable suspension. Prescribing Information. North Chesterfield, VA: Indivior Inc.
- INVEGA SUSTENNA® Current Prescribing Information. Titusville, NJ: Janssen Pharmaceuticals, Inc.
- INVEGA TRINZA® Current Prescribing Information. Titusville, NJ: Janssen Pharmaceuticals, Inc.
- ABILIFY MAINTENA® Current Prescribing Information. Rockville, MD: Otsuka America Pharmaceutical, Inc.
- Robinson DG, Suett M, Wilhelm A, et al. Patient and healthcare professional preferences for characteristics of long-acting injectable antipsychotic agents for the treatment of schizophrenia. Adv Ther. 2023;40(5):2249-2264.
- Højlund M, Correll CU. Switching to long-acting injectable antipsychotics: pharmacological considerations and practical approaches. Expert Opin Pharmacother. 2023;24(13):1463-1489.
- Perlstein I, Meyer J, Yue Z, et al. Switching patients with schizophrenia from intramuscular paliperidone palmitate once monthly to TV-46000, a long-acting subcutaneous antipsychotic: population pharmacokinetic-based strategies. Presented at: Psych Congress Elevate; May 30-June 2, 2024; Las Vegas, NV.



